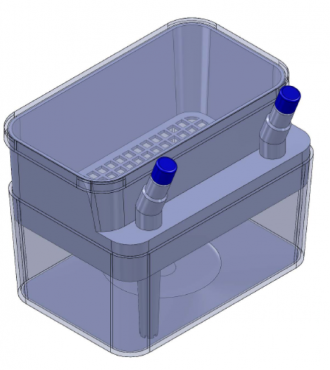
Allograft Preservation Chamber
Problem Statement
People get their bones damaged or deteriorated as a result of injury, disease, osteoarthritis, and old age. These injuries can have a negative impact on the rest of the patient’s life if the problem remains untreated. Often the most effective way of treating these individuals is through a transplant from a healthy donor. In most cases the donor has passed away, but the part of the body needed by the recipient is still in good condition. This process is called allotransplantation, in which a tissue graft (otherwise known as the allograft) is received from a donor of the same species as the recipient but not genetically identical.
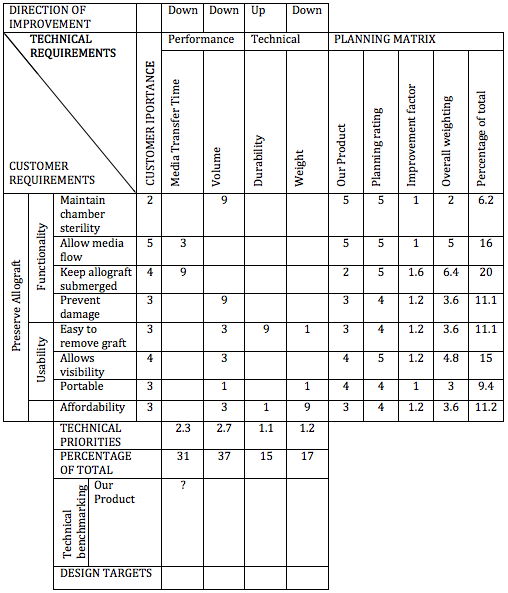
An allograft preservation system needs to be designed that is affordable, non-toxic, and safe to contain the allograft inside until it is to be transplanted into the body of the recipient. Factors that must be taken into consideration for the system to work efficiently include media, preservation method (deep freeze, freeze-dry, etc.), temperature, size, and filters used to bring substances in and out of the medium. The system must be able to remove old tissue culture media and add new media sterilely.
Video Introduction
Allograft Transplantation
Allografts are tissues, cells, or organs which are transplanted from one member to another of the same species, in a process known as allotransplantation. It is generally more advantageous than using autografts because it does not require as much surgery in the process. However, allotransplantation could have some disadvantages, in that the donor's allograft could be infected or contaminated by a disease.
The process of allotransportation involves removing the tissue that needs to be transplanted from the donor (usually already dead, though in some cases it may be a living donor) and inserting it into a preservation chamber where it is to be kept and processed until it is ready to be given to the recipient. The Food and Drug Administration regulates the banks that these allografts are sent to, to ensure that they will be suitable for donating.
Schedule and Minutes
Designs
Design Outline
The current design for the apparatus involves a main media chamber housing the allograft in a mesh bag to act as a coarse filter and a simple removal method. The lid of the container is placed on top of the main chamber, and includes an ingoing and outgoing port for media, the latter of which includes a tube going to the bottom of the chamber so media is removed from the bottom.
Background Research
Sterilization
Media is to be sterilized through filtration membranes. These membranes are made of a variety of materials, including mixed esters of cellulose (MCE), polyvinylidene fluoride (PVDF), surface-modified polyethersulfone (PES), polytetrafluoroethylene (teflon), and polyethylene. Typical pore size for sterilization is 0.22 µm, and can allow a flow rate of 18 µL/min/cm2.More info
Media Transfer
There are a wide range of possible methods for transferring media to and from the allograft housing. Media can be inserted either through gravity, by placing the container of new media above the allograft housing, or through a pump. Removal of media will require use of a pump. The simplest and cheapest variant is a bulb pump, which is manually operated, and may be valuable in testing. More sophisticated, mechanically automated solutions include piston pumps and peristaltic pumps, as used in hemodialysis machines. View the pumps page here.
Valves will also be an essential tool in regulating the transfer of media, controlling the flow rate of media once it comes out of the chamber. There are several different types of valves that could be examined for this design. View the valves page here.
The exact process used for media transfer may be based on an existing fluid handling system.
For controlling the different types of media coming out of the chamber, a valve manifold serves as an ideal solution. Several different types of existing manifold systems for different numbers of ports can be viewed here. A flow meter can also be used to work similarly to measure and control the rate of flow for a particular fluid.
Manufacturing
The allograft apparatus is to be constructed out of plastic through injection molding. Additional components such as filtration membranes can be attached through ultrasonic welding.
An alternate method to designing out own apparatus for the two chambers and relying on manufacturers to put it together, is to search for and buy an already existing rectangular, clear-hard plastic bottom chamber, if it is manageable. If so, a reasonably similar solution to the one that we have already designed would allow us to use our resources more efficiently. Some possible resources to find such solutions include:
Fisher Scientific - Corning *CELLSTACK Culture Chambers
Incubator Culture Chamber (for best pictures click here)
These are not the most optimal solutions for us, but it's a start…