WELCOME! Hi My Name is Ashley Kim. I am a senior majoring in chemical engineering.
For ChE 141 (heat and mass transfer), I used COMSOL Multiphysics to simulate the heat transfer through an insulated pipe with various conditions.
The following picture
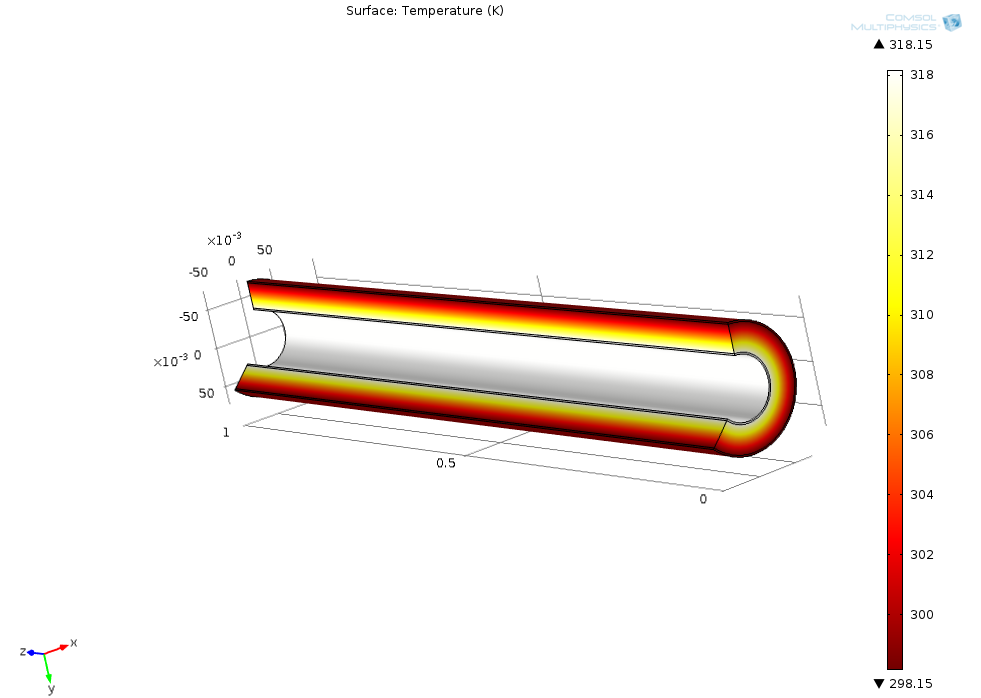
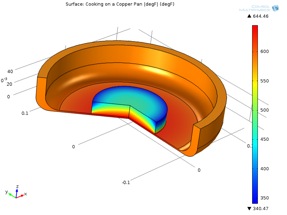
For the summer program at Cooper Union, I was a teaching assistant for the chemical engineering group. One of our focus was food science and how heat transfer is used in cooking. One of the projects that my high schoolers did was to use COMSOL Multiphysics to simulate cooking of a hamburger on a pan. Here is the capture of the model that we build of a hamburger patty on a pan
For PChem, One of the projects that I did with my partner, Dhongik Yoon, was to look at relative stabilities of carbocations using Spartan.
Here are some of the samples of the models that I did in spartan:
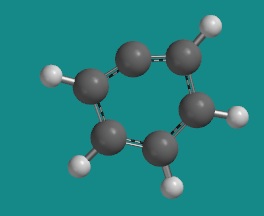 Benzene
Benzene
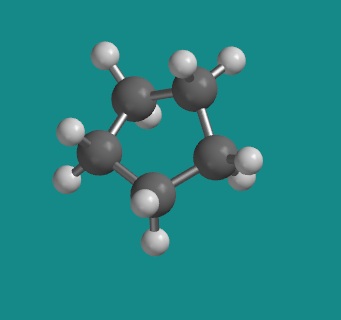 1,3-cyclopentane
1,3-cyclopentane
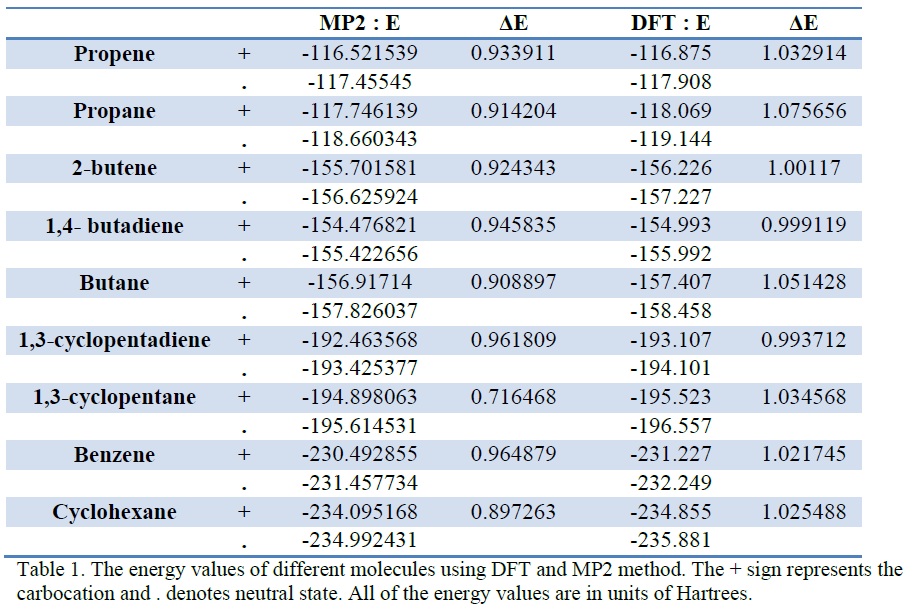
Results The results that were obtained using Spartan is shown in Table 1. The result calculated with DFT method actually came out as expected because the ΔE of a molecule with conjugated double bond was less than that of a molecule with only single bond. For example, the ΔE of propene was smaller than that of propane. Since lower energy is inversely proportional to relative stability, it was expected that the carbocation molecule of the double bond is more stable than that of single bond. The calculation results matched the expected outcome.However, as mentioned earlier, the results calculated with MP2 showed the direct opposite. This is due to the fact that the singlet multiplicity was used for the calculation of carbocation molecules. Considering results with DFT method only, the difference in ΔE between the ΔE of benzene and that of cyclohexane was the lowest of all. This might be because the stabilizing effect due to the resonance effect of the benzene molecule itself is so strong compared to that due to the conjugated double bonds. Also, the difference in ΔE between the ΔE of 1,4-butadiene and that of butane was lower than the difference in ΔE between the ΔE of 2-butene and that of butane. It is because the stabilizing effect due to the conjugated double bonds increases according to the numbers of double bonds present in one molecule. The triplet was chosen for the multiplicity option for carbocation. Because the total number of electrons present in one carbocation molecule is odd, the multiplicity should not be singlet for obvious reason. Method of trial and error was used to determine whether doublet or triplet should be used. And when the doublet was used, an error came up stating that the charge multiplicity is invalid – and when the triplet was used, the calculations were done without any difficulties.
Here is the Camaro Solidworks that I did for EID 103